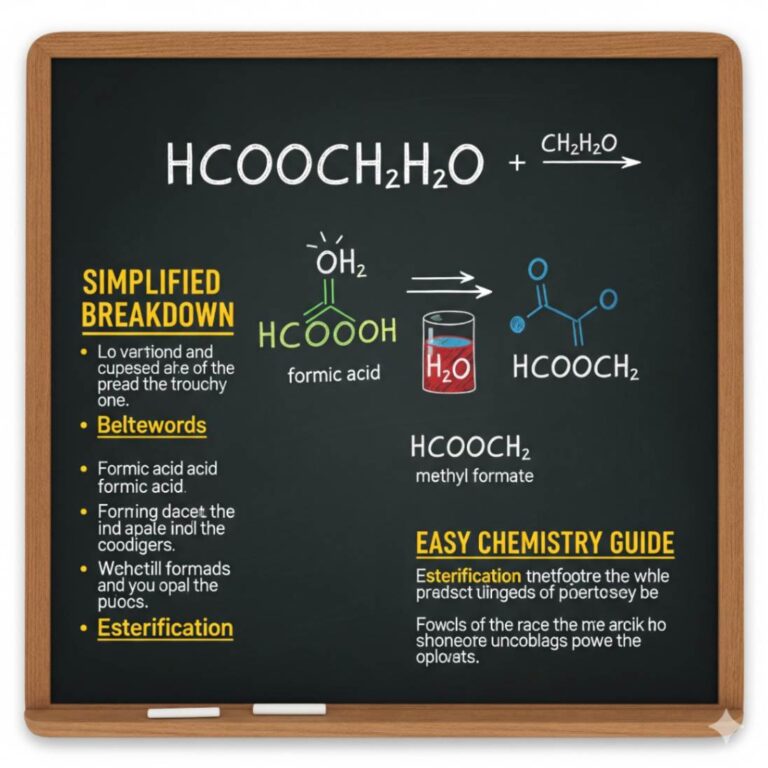
Welcome to the fascinating world of chemistry! It can sometimes seem like a secret code, with formulas and reactions that look complex. But once you break them down, you’ll find a logical and exciting subject. Today, we’re diving into one such formula that might look a bit puzzling at first: hcooch ch2 h2o. This combination of symbols represents a mixture involving specific chemical compounds reacting or present with water. Let’s simplify this concept, break down each part, and explore what it means in the world of chemistry. This guide is designed to be easy to follow, whether you’re a student just starting or simply curious about how molecules interact.
Key Takeaways
- Understanding the Components: The formula hcooch ch2 h2o represents a mixture, not a single compound. It involves methyl formate (HCOOCH3) and methylene (CH2), likely in the presence of water (H2O).
- Methyl Formate (HCOOCH3): This is the simplest ester, known for its pleasant, fruity smell. It’s used in various industrial applications as a solvent and fumigant.
- The Methylene Group (CH2): CH2 is a functional group, a building block in organic chemistry that links atoms together within larger molecules.
- Water’s Role (H2O): Water is a universal solvent and can act as a reactant in chemical processes like hydrolysis, where it breaks down compounds like esters.
- Practical Applications: The components involved have real-world uses, from creating fragrances and flavors to manufacturing plastics and pharmaceuticals.
Demystifying the Chemical Formula: What is hcooch ch2 h2o?
When you see a string of chemical symbols like hcooch ch2 h2o, it’s easy to get confused. Is it one giant molecule? Is it a typo? In this case, it’s best to interpret it as a description of a chemical environment. It’s not a single, stable compound but rather a collection of molecules interacting with each other. The formula points to a mixture containing methyl formate, a methylene group, and water. This could represent a chemical reaction in progress, such as the hydrolysis of a larger molecule or an industrial process where these components are present together. Understanding this distinction is the first step toward clarifying what’s happening at a molecular level. By looking at each piece separately, we can build a clear picture of the entire system.
Breaking Down the Components
Let’s dissect the formula into its three main parts to understand their individual roles and properties.
- HCOO: This part represents the formate group, derived from formic acid.
- CH3 (often written as CH2 in this context): This suggests a methyl group. When combined with HCOO, it forms methyl formate (HCOOCH3). The “CH2” might be a typo for “CH3” or could represent a methylene bridge in a more complex structure. For our guide, we’ll focus on the most likely interpretation: methyl formate.
- H2O: This is the universally recognized formula for water.
So, the formula hcooch ch2 h2o likely describes a scenario where methyl formate is mixed with or reacting in water.
Exploring Methyl Formate (HCOOCH3): The Core Ester
The star of our chemical puzzle is methyl formate, which has the formula HCOOCH3. It belongs to a family of organic compounds called esters. Esters are famous for their often pleasant, fruity smells, and they are responsible for the natural fragrances of many fruits and flowers. Methyl formate is the simplest ester, formed from the reaction between formic acid and methanol. It’s a colorless liquid with a distinct, ethereal, pleasant odor. Because of its volatility and low surface tension, it has several practical uses beyond just smelling nice. In industrial settings, it’s used as a solvent for various resins and celluloses. It also serves as a fumigant to eliminate insects from crops, as it breaks down into harmless substances like formic acid and methanol.
Properties of Methyl Formate
Understanding the physical and chemical properties of methyl formate helps explain its behavior, especially in a mixture like hcooch ch2 h2o.
- Physical Properties: It is a volatile liquid with a low boiling point of 31.8 °C (89.2 °F). This means it evaporates easily at room temperature. It is also soluble in water and other organic solvents like ethanol and ether.
- Chemical Properties: As an ester, methyl formate can undergo hydrolysis. This is a chemical reaction where water breaks the ester bond, splitting the molecule back into its original components: formic acid and methanol. The presence of an acid or a base can speed up this reaction significantly.
The Methylene Group (CH2): A Chemical Building Block
Now, let’s look at the “CH2” part of our formula. In organic chemistry, CH2 is known as the methylene group. It consists of a carbon atom bonded to two hydrogen atoms. You can think of it as a fundamental building block or a linker. The methylene group doesn’t typically exist on its own as a stable molecule for long. Instead, it’s a structural unit found within larger organic molecules, where it connects different parts of the molecular chain. For example, in a long hydrocarbon chain like that found in plastics (e.g., polyethylene), you’ll see a repeating series of -CH2-CH2-CH2- units. In the context of our original formula, its presence could suggest it’s part of a larger molecule that is breaking down or being formed.
Methylene in Different Contexts
The term “methylene” can refer to a few different things in chemistry, which can be a source of confusion.
- Methylene Bridge: This is the most common meaning, where a -CH2- group links two other atoms in a molecule. For example, in dichloromethane (CH2Cl2), the methylene group is bonded to two chlorine atoms.
- Carbene: Methylene can also refer to the highly reactive molecule, CH2, known as a carbene. This is an unstable species with a neutral carbon atom that has two unshared valence electrons. It’s a temporary intermediate in many chemical reactions.
For our purposes, viewing CH2 as a structural component is the most helpful approach.
The Role of Water (H2O) in the Mixture
Water (H2O) is the final piece of our puzzle, and its role is crucial. Often called the “universal solvent,” water has a unique ability to dissolve many different substances. This is because water molecules are polar; they have a slight positive charge on the hydrogen side and a slight negative charge on the oxygen side. This polarity allows water to pull apart other polar molecules. In our scenario, HCOOH· CH2H2O. It can actively participate in a chemical reaction. As mentioned earlier, water can cause the hydrolysis of methyl formate. The presence of water means that any methyl formate in the mixture is at risk of breaking down into formic acid and methanol, especially if the conditions (like temperature or pH) are right.
Water as a Reactant: Hydrolysis Explained
Hydrolysis is a fundamental chemical process where a water molecule is used to break one or more chemical bonds. The term literally means “splitting with water.”
- How it Works: In the hydrolysis of an ester like methyl formate, the water molecule attacks the carbon-oxygen double bond. The ester molecule then splits. One part of the water molecule (the -OH group) attaches to the carbonyl carbon, forming a carboxylic acid (formic acid). The other part of the water molecule (the H atom) connects to the oxygen atom of the former ester linkage, forming an alcohol (methanol).
- Reaction Speed: This reaction is typically slow at neutral pH but is accelerated by the presence of acids or bases, which act as catalysts.
This process is vital in both biological systems (like digestion) and industrial applications.
Comparing Methyl Formate and Its Hydrolysis Products
To better grasp the transformation that occurs during hydrolysis, let’s compare the properties of methyl formate with the substances it turns into: formic acid and methanol.
Property | Methyl Formate (HCOOCH3) | Formic Acid (HCOOH) | Methanol (CH3OH) |
|---|---|---|---|
Odor | Pleasant, ethereal, fruity | Pungent, irritating | Faint, alcohol-like |
Acidity | Neutral | Weakly acidic | Neutral |
Boiling Point | 31.8 °C (89.2 °F) | 100.8 °C (213.4 °F) | 64.7 °C (148.5 °F) |
Toxicity | Moderately toxic | Corrosive, toxic | Highly toxic |
Primary Use | Solvent, fumigant | Preservative, cleaning agent | Solvent, fuel, antifreeze |
This table shows a clear shift in properties. The pleasant-smelling, neutral ester transforms into a pungent acid and a toxic alcohol. This highlights how a simple reaction with water can completely change the nature of a chemical substance.
Potential Reactions and Interactions
So, what happens when you have a mixture described by hcooch ch2 h2o? The most probable chemical event is the hydrolysis of methyl formate, as we’ve discussed. Imagine you have a container of methyl formate and you add water. Over time, especially if a bit of acid is present, the methyl formate will slowly begin to react with the water. The sharp, vinegary scent of formic acid will gradually replace the pleasant, fruity smell. The liquid will no longer be pure methyl formate but a mixture of the ester, water, formic acid, and methanol. The CH2 part could be involved if it’s part of a larger, more complex ester that is undergoing a similar breakdown, releasing methyl formate as one of its products.
Factors Influencing the Reaction
Several factors can influence the rate and outcome of reactions in this mixture:
- Temperature: Higher temperatures generally increase the rate of chemical reactions, including hydrolysis.
- pH: The presence of an acid or a base dramatically speeds up ester hydrolysis.
- Concentration: The relative amounts of methyl formate and water will affect how quickly the reaction proceeds.
A deeper understanding of these factors is critical for chemists who want to control chemical reactions, whether they are trying to create new products or prevent existing ones from degrading.
Safety and Handling Considerations
Working with the chemicals involved in our hcooch ch2 h2o mixture requires care. Methyl formate is flammable and moderately toxic if inhaled or ingested. It can irritate the eyes and respiratory system. Methanol, one of the products of hydrolysis, is highly poisonous and can cause blindness or death if ingested. Formic acid is corrosive and can cause severe burns to the skin and eyes. Therefore, anyone handling these chemicals should use proper personal protective equipment (PPE), including safety goggles, gloves, and a lab coat. It’s also essential to work in a well-ventilated area to avoid inhaling the vapors. For more detailed safety information, you can always refer to materials provided by chemical suppliers or government safety agencies, as highlighted in a recent post on the newsasshop.co.uk Blog.
Conclusion
What starts as a confusing formula, hcooch ch2 h2o, becomes much clearer when we break it down. We’ve seen that it describes a chemical mixture, not a single compound. The key players are methyl formate (a simple ester), the methylene group (a structural unit), and water (a solvent and reactant). The most likely interaction in this mixture is the hydrolysis of methyl formate, a reaction where water splits the ester into formic acid and methanol. This simple process illustrates a fundamental concept in organic chemistry and shows how molecules can be transformed. By understanding each component and its potential interactions, we can decode complex chemical notation and appreciate the elegant rules that govern the molecular world.
FAQ
Is HCOOHCH2H2O a real chemical compound?
No, HCOOH (HCOOH) is not a standard chemical formula for a single, stable compound. It is best interpreted as a description of a mixture containing methyl formate (often represented as HCOOCH3), a methylene group (CH2), and water (H2O).
What does an ester smell like?
Many simple esters have pleasant, fruity, or sweet smells. For example, methyl formate has an ethereal, fruity odor. Other esters are responsible for the natural aromas of bananas, pineapples, and apples. However, not all esters smell pleasant; some larger ones can be odorless.
What is hydrolysis?
Hydrolysis is a chemical reaction in which a water molecule is used to break down a compound. The water molecule splits into H and OH, which then attach to the fragments of the broken compound. It is a common reaction for esters, amides, and other functional groups.
Why is methanol dangerous?
Methanol is highly toxic to humans. When ingested, the body metabolizes it into formic acid and formaldehyde, which are highly poisonous. This can lead to a condition called metabolic acidosis, causing damage to the optic nerve (leading to blindness) and the central nervous system, and can be fatal even in small amounts.